Metals Tend to Form Cations or Anions
A simplistic model of the atom Bohrs model is the nucleus at the center of a series of concentric spheres. It is easiest to achieve noble gas configurations by removing valence electrons from metals which have few valence electrons and adding them to nonmetals which have many valence electrons.

Cation Vs Anion Definition Chart And The Periodic Table Technology Networks
Metals generally have 12 or 3 valence electrons which can be lost to attain octet configuration and thus they form cations.
. Identify the reason why metals tend to form cations and nonmetals tend to form anions when these elements exist in a compound. Metals generally lose electrons to achieve a noble gas configuration when they have one two or three valence electrons and thus become cations. Do metalloids form cations or anions.
1 metals have low lonization energies II nonmetals have high ionization energies III cations are have smaller radii. Cations are positively charged ions. For example in NaCl the cation Na is formed by loss of an electron from the group 1 metal sodium.
When they have five six or seven valence electrons nonmetal elements tend to gain electrons and thus become anions. Yes in general the cation is the metal and the anion is the non-metal. View the full answer.
Groups 1 and 2 are called the alkali metals and alkaline Earth metals respectively. The metallic elements generally form cations and the non-metal elements typically form anions. Non metals have 4 5 6 or 7 valence electrons and can only gain electrons to attain octet configuration and thus they form anions.
Keep in mind that metals tend to have lower ionization energies and are much more likely to lose electrons making them cations as they become positively charged while non-metals are typically more electronegative and pull electrons towards themselves making them anions as they become. Most other metals form cations eg. Metals like to lose valence electrons to form cations to have a fully stable octet.
Metal elements form positively charged ions called cations because they are located on the left side of the periodic table. Anions are negatively charged ions. These elements all have valence electrons in an s orbital.
Why do metals always form cations. Energy is released when electrons are removed from metal ions. All metals will form cations.
Metals form cations bc they only need to lose 1 or two electrons. By writing the name of the cation followed by the name of the anion. While the chlorine anion Cl- is formed by the gain of an electron by the group 7 non-metal chlorine atom.
Posted by Ned Kaufman. Nonmetals will form anions except for the noble gases which are not reactive. Anions tend to be ____ and cations tend to be ____.
The electron affinity of metals is lower than that of nonmetals. Iron silver nickel whilst most other nonmetals typically form anions eg. 0 followers 0 following Joined September 2020.
Home Community Do metalloids form cations or anions. Nonmetals like to gain electrons to form anions to. They absorb energy endothermic to lose electrons.
Downvote Cations Anions Ions Chemistry. It can be possible to predict whether an atom will form a cation or an anion based on its position on the periodic table. Metals form cations because of their electronic structure.
Nonmetals form anions bc they are closer to a full valence shell and they want to gain electrons. The cation is the same as the metal name the anion is the name of the nonmetal w the suffix changed to -ide Sn and Pb from the p block will _____ Form more than one type of ion and behave like transition metals. Generally metals tend to lose electrons to form cationsthat shows they have lower.
The protons and neutrons are in the nucleus and the electrons occupy the surrounding spheres energy levels. Otherwise more energy would be required for them to gain the necessary electrons. Question 6 1 point Why do metals tend to form cations while nonmetals form anions.
Do metals form anions or cations quizlet. Metals tend to form cations and lose. Metal elements form positively charged ions.
Why do metals form cations. Halogens always form anions alkali metals and alkaline earth metals always form cations. When they lose electrons they become positive and cations are positively charged ions.
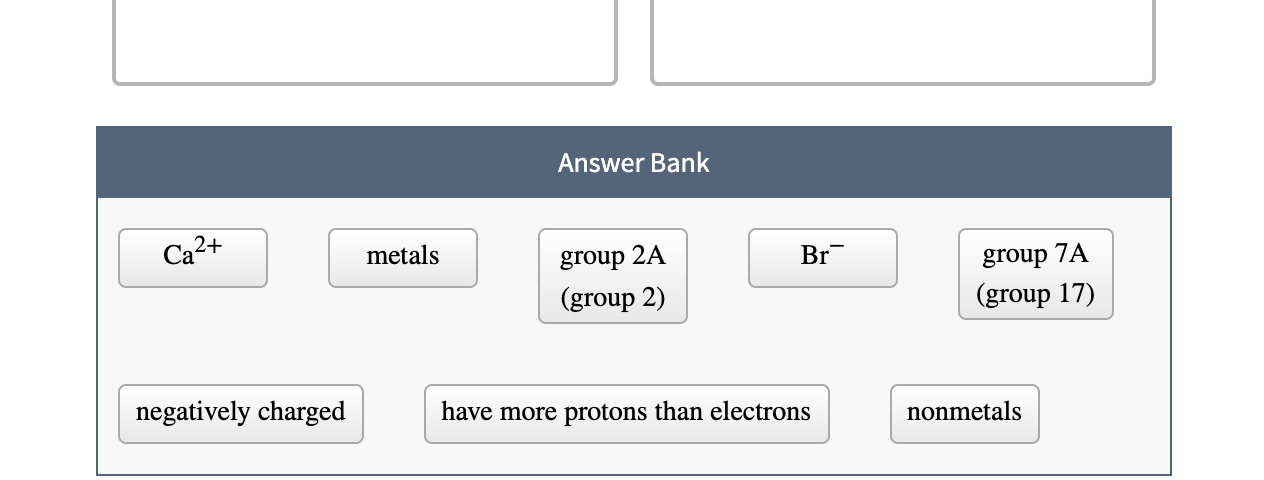
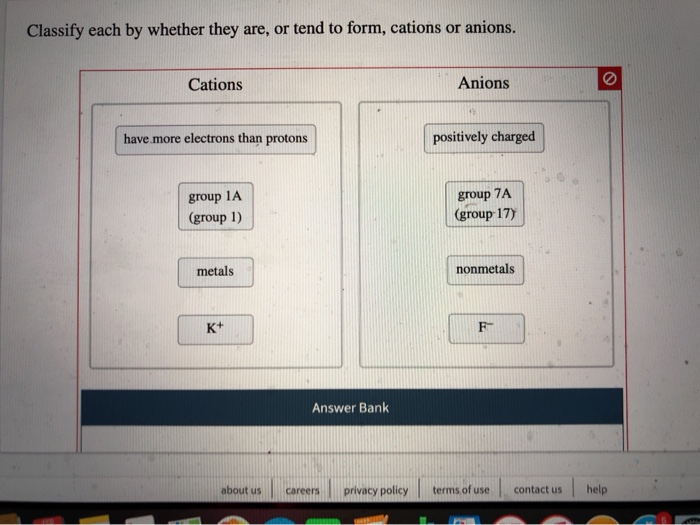
Solved Classify Each By Whether They Are Or Tend To Form Chegg Com

Solved Classify Each By Whether They Are Or Tend To Form Chegg Com

Solved Question 6 1 Point Why Do Metals Tend To Form Chegg Com
No comments for "Metals Tend to Form Cations or Anions"
Post a Comment